It is recommended that pupil diameter measurement be performed as part of every thorough eye check-up since it may detect significant retinal and neuro-ophthalmic disorders. When used in conjunction with a thorough clinical examination, this test may assist in the diagnosis of pupillary response in traumatic brain injury and treat many of these disorders at the primary care level. The purpose of this article is to discuss the most often observed pupil problems and how doctors might identify them via regular pupil testing.
Neuroanatomy
A strong working understanding of the anatomy of the pupillary light reflex and the autonomic innervation of pupil reactivity is required for meaningful interpretation of pupillary results. Parasympathetic nerves control the pupillary light reflex and immediate reactions. The pupillary light response is composed of an afferent route and an efferent pathway. Incoming light is transmitted through the retina’s photoreceptors, through the optic nerve to the chiasm and optic tract, which then separates from the tract just anterior to the lateral geniculate body (LGN) before traveling to the midbrain and bilaterally projecting to the pretectal nuclei. The efferent pathway transmits information from the pretectal nuclei to the thalamus. CN III contains two Edinger-Westphal nuclei, which serve as the starting point for the efferent pathway. Pupil fibers synapse with pretectal nuclei in the midbrain and travel to the two Edinger-Westphal nuclei of the oculomotor nerve (CN III), where they form a connection with the efferent pathway.
It is possible to objectively test the integrity of the afferent route by having a doctor measure pupil size and examine both the direct and consensual light responses resulting from this neuroanatomy. Suppose the right eye responds directly (and the left eye responds consensually). In that case, the right afferent route is in good working order, for example. Additionally, this is why a lesion of the optical nerve or optical track does not result in an increase in the size of the pupil in either eye when a lesion occurs in the optic nerve or optical tract. Once in orbit, efferent pupil fibers travel with CN III back to the eyeball, where they synapse in the ciliary ganglion, with 3 percent of post-ganglionic fibers innervating the iris sphincter muscle (which allows for miosis) and the remaining 97 percent innervating the ciliary body (which controls blinking) (which allows for accommodation).
There are three neurons that make up the oculo-sympathetic nerve that innervates the eye. The first-order neuron originates in the posterior hypothalamus. It descends via the brainstem to synapse in the ciliospinal center of Budge, which is located between the levels of the eighth cervical fourth thoracic vertebrae (C8-T4). Upon leaving the spinal cord, the second-order neuron continues on its path, passing across the apex of the lung and synapsing with the superior cervical ganglion. After being formed by third-order neurons, post-ganglionic axons emerge from the superior cervical ganglion and travel along the course of the internal carotid artery through the cavernous sinus, where they meet up with the ophthalmic division of the trigeminal nerve (V1) and the ophthalmic artery to continue their journey to the eye. In the case of the ophthalmic artery, neurons go on to innervate Mueller’s muscle, which is responsible for eyelid control; in the case of V1, neurons continue to innervate the iris dilation muscle, which is responsible for mydriasis.
The Swinging Flashlight Test
Due to the intensity of the light, the swinging flashlight test is preferred over the handheld penlight when diagnosing afferent pupillary defects. Clinicians should perform the test in a dark room with a transilluminator or the light from a binocular indirect ophthalmoscope, which is preferred over the handheld penlight when diagnosing afferent pupillary defects. Using the same eye, the intensity of the direct pupillary response is compared to that of the consensual pupillary response. When the consensual response in the afflicted eye is larger than the direct response in the other eye, the patient has a relative afferent pupillary deficit (RAPD), also known as an APD or Marcus Gunn pupil, which indicates injury at or anterior to the lateral gaze nucleus (LGN).
The damage must be unilateral or asymmetric to induce an RAPD, which might occur in the case of severe retinal disease, optic nerve illness or impairment, or a lesion behind the eye. Because there is no such thing as a bilateral APD, a severe but bilaterally equal condition will not result in an RAPD. Furthermore, even in the most severe cases, an RAPD cannot be induced by ocular media or refractive abnormalities. It is not always the case that diminished visual acuity in one eye is associated with an RAPD; nonetheless, doctors should always seek one when severely reduced acuity is in one eye.
The defect may be quantified and a grade awarded when a neutral density filter is placed over the good eye. For the most part, neutral density filters come in various densities, with the most useful densities being the ones that range between 0.33 and 0.66 log units and 0.90 and 1.2 log units, respectively. Grading may be useful for spotting minor flaws and tracking the evolution of a condition. For pupil diagnostics, newer, high-definition equipment is now available, enabling objective, comprehensive, and quantitative measurements of both the direct and consensual light responses to be taken.
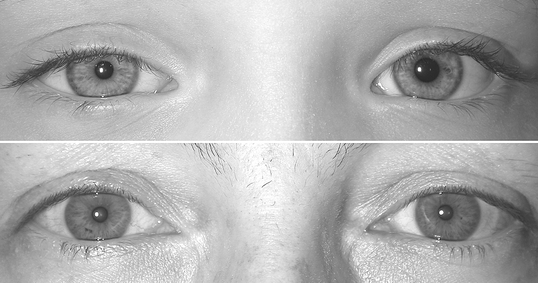
Evaluating Pupil Shape and Size
The iris and pupil should be in perfect alignment. With the parasympathetic innervation (Iris sphincter) governing pupil size and the quantity of light that can pass through the eye, the sympathetic innervation (Iris dilator) is less strong in controlling pupil size.
The size of a patient’s pupil fluctuates regularly due to variations in light and accommodation. The patient’s pupil diameter should be measured as part of the first phase of pupil testing to check for any signs of anisocoria. Pupil diameter ranges from 1.0mm to 10mm in adults and decreases as one age due to senile miosis in the latter years of life. Clinically significant is a discrepancy between the two eyes of at least 0.4mm. Only one of the patient’s pupils is affected when they have anisocoria. The underlying cause might be physiologic (which affects around 20% of healthy individuals), pharmacologic, or pathologic.
Further evaluation of anisocoria should include reassessing pupil size in both bright and dim light to segregate the parasympathetic and sympathetic pathways. The bigger pupil indicates parasympathetic denervation if the difference in pupil diameters is higher under bright light. If the difference is higher under dim lighting, the smaller pupil is abnormal because of aberrant sympathetic innervation.
Patients who have recently taken or come into contact with drugs or substances that may alter the size of their pupils or who have a history of recent trauma or surgery should be thoroughly and thoughtfully documented in each case of suspected pupil abnormality. To get a clearer idea of the beginning and duration of a condition, doctors might consult old photographs or a patient’s driver’s license.
All illumination settings should be consistent if the anisocoria is physiologic in origin. No more than 1mm of physiologic anisocoria exists, and it may fluctuate during the day and even swap eyes. If physiologic anisocoria is detected, there is no need for additional pharmacological examination. However, doctors should always rule out other neuropathologies.
